843
Views & Citations10
Likes & Shares
Atrial
fibrillation is the most common arrhythmia associated with significant
mortality and morbidity secondary to thrombo-embolism. To prevent this
thrombo-embolism oral anticoagulation therapy is the recommended treatment. In
patients with contraindications to oral anticoagulation therapy, percutaneous
left atrial appendage occlusion device is indicated. TEE is essential to guide
in all the stages of LAA device deployment.
Keywords: Atrial fibrillation, Transoesophageal echocardiography, Left atrial appendage occlusion device, Amplatazer cardiac plug
INTRODUCTION
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia encountered in clinical practice. The incidence is around 3-5% in the age group of 65-75 years and increasing to >8% in patients more than 80 years. It is associated with increased mortality and morbidity. One of its most devastating complications is stroke secondary to thromboembolism. Overall, AF accounts for 15-20% of strokes in the general population and for up to 30% in patients over the age group of 80 years. If untreated, the risk of stroke is 3-5% per year in patients with non-valvular AF (NVAF) [1-3]. To prevent this, oral anticoagulation (OAC) is the treatment of choice [4]. This anticoagulant therapy has been proven to effectively prevent thromboembolic strokes, but increases the risk of serious bleeding, which has an incidence of 2-4% per year [5]. In addition, these drugs have a small therapeutic window, several foods and drug interactions and require frequent blood testing, which makes this therapy inconvenient to many patients. Novel OACs are also associated with increased bleeding and gastric intolerance [6]. So far, neither pharmacological cardioversion nor radio-frequency catheter ablation has been proven to eliminate the indication for long-term OAC therapy [7,8]. Hence, alternative treatment options are essential. Left atrial appendage (LAA) occlusion device is one such option in patients where OAC is contraindicated. CHA2DS2-VASc score is used to assess the risk of stroke in patients with AF. If the score is >2 it is an indication to start OAC therapy [9]. HAS-BLED is a scoring system developed to assess 1 year risk of major bleeding in patients taking anticoagulants with atrial fibrillation. If the score is >3 then the patient is at high risk for bleeding, one has to be cautious [10].
ANESTHETIC CONSIDERATIONS AND DISCUSSION
Atrial fibrillation is a common arrhythmia frequently seen during the perioperative period in patients undergoing surgery. Usually new onset atrial fibrillation is uncommon during the perioperative period. The rapid rates of new onset AF or pre-existing AF may be precipitated by several factors, including sepsis, dyselectrolytemia like hypokalemia, hypomagnesimia and acid-base abnormalities, pulmonary complications like pulmonary embolism, hypoxia, hypovolemia, myocardial ischemia, etc. [11,12]. Elderly patients, diabetics, hypertensive, valvular heart disease, ventricular and atrial enlargement, thyrotoxicosis, diastolic heart failure is at higher risk of developing atrial fibrillation. Roger et al reported that the overall incidence of supraventricular tachycardia was estimated to be less than 1% and in those with an SVT, the incidence of AF and atrial flutter was 30% and 12%, respectively with only 20% of
NON-PHARMACOLOGICAL MANAGEMENT
A wide variety of non-pharmacological approaches now exist for managing AF and provide rhythm or rate control when drug treatment has failed. To control ventricular rate transcatheter AV junctional ablation with permanent pacemaker implantation or radio-frequency transcatheter AV junction modification. To restore sinus rhythm device therapy like atrial pacing or atrial defibrillators, ablation therapy like percutaneous pulmonary vein isolation or radio-frequency trigger or substrate ablation, operative procedure like Maze procedure, pulmonary vein isolation or His bundle ablation. To prevent thromboembolism there are left atrial appendage occlusion devices like Watchman, AMPLATZER cardiac plug and COHEREX WaveCrest are available. Surgical/thoracoscopic LA appendectomy and the epicardial LARIAT suture delivery device are also described. WATCHMAN is an FDA approved device for NVAF with contraindication for OAC therapy.
Anesthetic considerations avoid drugs causing tachycardia like ketamine, Glycopyrolate and pancuronium, avoid lighter plans of anesthesia, adequate analgesia is very essential-opioids like fentanyl preferred as they reduce the heart rate, induction agent either etomidate or propofol may be preferred, relaxant vecuronium, rocuronium or atracurium can be used. A defibrillator should be checked and kept ready. Amiodarone, β-blockers, calcium channel blockers and digoxin should be available. Hypokalemia, hypomagnesemia, hypoxia and acidosis, should be preoperatively corrected. ECG, BP monitoring- invasive BP preferred and central venous access should be secured.
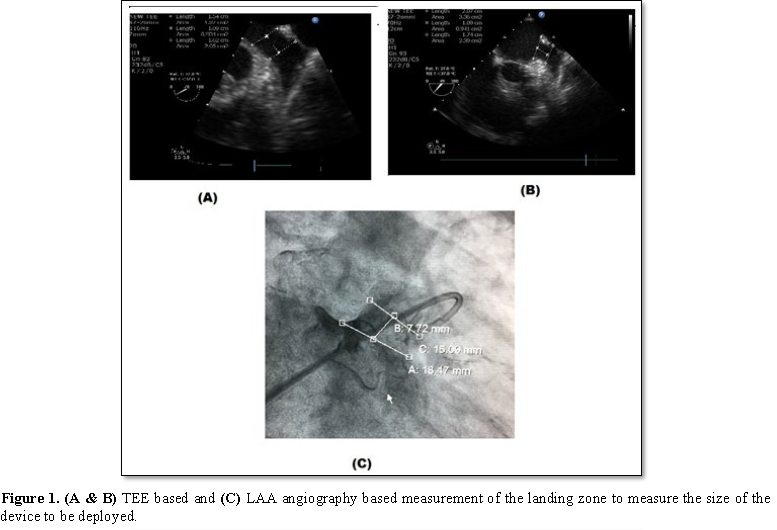
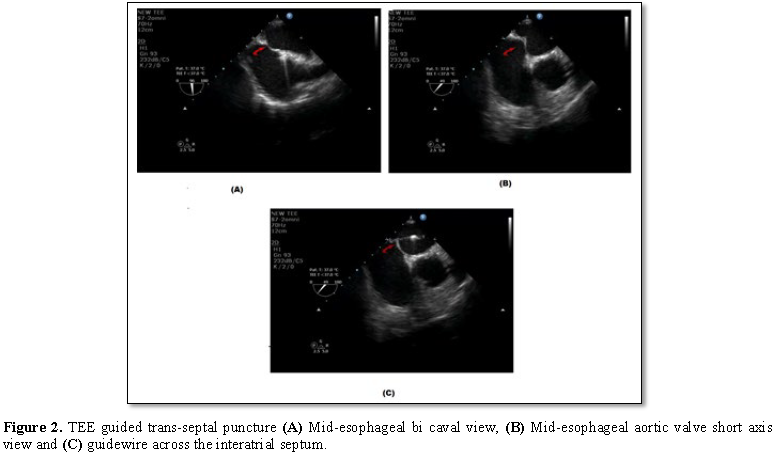
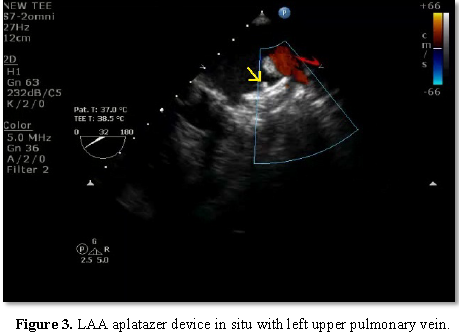
Transoesophageal echocardiography (TEE) plays a very important role in all the stages of LAA occlusion device therapy. Before deployment, TEE is used to rule out any thrombus in the left atrium or LAA as it is a contraindication for deployment of the device. The presence of spontaneous echo contrast is not a contraindication. Pre procedure, one has to define the morphology and dimensions of LAA. Morphologically, there are four types of LAA – chicken wing, cauliflower, windsock and cactus type. One has to assess the shape and size of the ostium, the width of the “landing zone” (area within the LAA where the device will be positioned), the length of the LAA, and, if possible, the number, shape and location of the lobes. Lobes are better defined with cardiac computed tomography or LA angiography. 3D echocardiography will also better define the shape and lobes of LAA, but due to irregular heart rate, artifacts are common. Live 3D will be more useful. Baseline left atrial dimensions is measured in anteroposterior and craniocaudal plane using mid-esophageal views at sector angle 0 and 120 degrees respectively. Ostial dimensions are noted in four mid-esophageal (ME) views: (i) 0°-20° four chamber view with slight flexion or withdrawal to open the LAA, (ii) 45°-60° aortic valve (AV) short axis view (SAX), (iii) 90° apical two chamber view, and (iv) 120-135° long-axis view with probe turned counterclockwise to open the subsidiary lobes. While using the ACP, the size is determined by the width of the landing zone. The LAA neck width (Landing zone) is typically measured 10 mm distal to the LAA ostium (Figure 1). 3-5 mm more than the largest diameter should be used to size the device. LA inflow and outflow has to be assessed initially, which may get altered following the device deployment. In the mitral valve, presence of any regurgitation and its peak velocity should be noted. The device is deployed using vascular access through the right femoral vein and then entering the left atrium through the transseptal puncture. Most important step during the procedure is transseptal puncture. TEE is very useful in guiding the puncture. Unlike transseptal puncture for balloon mitral valvotomy, the puncture is made in the inferior and posterior part of the fossa ovalis to get better alignment with the axis of LAA. The orthogonal views ME AV SAX and bi-caval views are used to guide the cardiologist during trans septal puncture. While puncturing the septum, it should be away from the AV in ME AV SAX view and toward inferior vena cava in bi-caval view (Figure 2). After the transseptal puncture, the patient is haparinised to maintain an ACT>250 s. TEE and contrast angiography of the LAA (right anterior oblique 30°/cranial 30°) are used to measure the LAA dimensions (ostium, neck width and depth) based on these measurements, the size of the device is chosen. The positioning of the device in the LAA cavity is ensured by TEE and fluoroscopy; the axis of the device should be in alignment with the major axis of LAA. Once in position, the device stability is confirmed by a Tug test, and complete sealing is verified by color Doppler imaging with lower Nyquist limits (Figure 3). Finally, the device is released from the delivery cable and possible complications such as compression of LUPV, impingement on the mitral valve, residual leak and new-onset mitral regurgitation or regional wall-motion abnormality of the lateral wall due to LCX compression to be excluded.
CONCLUSION
The percutaneous LAA occlusion device has been shown to be a safe, efficacious and cost-effective strategy for stroke prevention in patients with NVAF with an increased risk of stroke and bleeding. TEE is useful to assess the suitability of the patient for device closure, to measure the size and select the device to be deployed, to guide during transseptal puncture and deployment of the device, to assess the stability of the device following deployment, and to rule out any complication following procedure and during long-term follow-up.
1.
Wolf PA, Abbott RD, Kannel WB
(1991) Atrial fibrillation as an independent risk factor for stroke: The
Framingham study. Stroke 22: 983-988.
2.
Furberg CD, Psaty BM, Manolio
TA, Gardin JM, Smith VE, et al. (1994) Prevalence of atrial fibrillation in
elderly subjects - The Cardiovascular Health Study. Am J Cardiol 74: 236-241.
3.
Go AS, Hylek EM, Philips KA,
Chang Y, Henault LE, et al. (2001) Prevalence of diagnosed atrial fibrillation
in adults: National implications for rhythm management and stroke prevention:
The Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) study. JAMA
285: 2370-2375.
4.
Camm AJ, Lip GY, De Caterina R,
Savelieva I, Atar D, et al. (2012) 2012 focused update of the ESC Guidelines
for the management of atrial fibrillation: an update of the 2010 ESC Guidelines
for the management of atrial fibrillation developed with the special
contribution of the European Heart Rhythm Association. Europace 14: 1385-1413.
5.
Connolly SJ, Ezekowitz MD,
Yusuf S, Eikelboom J, Oldgren J, et al. (2009) Dabigatran versus warfarin in
patients with atrial fibrillation. N Engl J Med 361: 1139-1151.
6.
Calkins H, Kuck KH, Cappato R,
Brugada J, Camm AJ, et al. (2012) 2012 HRS/EHRA/ECAS expert consensus statement
on catheter and surgical ablation of atrial fibrillation: Recommendations for
patient selection, procedural techniques, patient management and follow-up,
definitions, endpoints and research trial design. Europace 14: 528-606.
7.
Lockwood SM, Alison JF,
Obeyesekere MN (2011) Imaging the left atrial appendage prior to, during and
after occlusion. JACC Cardiovasc Imaging 4: 303-306.
8.
Wang Y, Di Biase L, Horton RP,
Nguyen T, Morhanty P, et al. (2010) Left atrial appendage studied by computed
tomography to help planning for appendage closure device placement. J
Cardiovasc Electrophysiol 21: 973-982.
9.
Olesen JB, Lip GY, Hansen ML,
Hansen PR, Tolstrup JS, et al. (2011) Validation of risk stratification schemes
for predicting stroke and thromboembolism in patients with atrial fibrillation:
Nationwide cohort study. BMJ 342: d124.
10.
Pisters R, Lane DA, Nieuwlaat R
(2010) A novel user-friendly score (HAS-BLED) to assess 1 year risk of major
bleeding in patients with atrial fibrillation: The Euro Heart Survey. Chest
138: 1093-1100.
11.
Nathanson MH, Gajraj NM (1998)
The peri-operative management of atrial fibrillation. Anesthesia 53: 665-676.
12.
Thompson A, Basler JR (2004)
Perioperative cardiac arrhythmias. Br J Anesth 93: 86-94.
13.
Wyse DG, Waldo AL, DiMarco JP,
Domanski MJ, Rosenberg Y, et al. (2002) A comparison of rate control and rhythm
control in patients with atrial fibrillation. N Engl J Med 347: 1825-1833.
14.
Hohnloser SH, Kuck KH,
Lilienthal J (2000) Rhythm or rate control in atrial fibrillation -
Pharmacological intervention in atrial fibrillation (PIAF): A randomised trial.
Lancet 356: 1789-1794.
15.
Carlsson J, Miketic S, Windeler
J, Cuneo A, Haun S, et al. (2003). Randomized trial of rate-control versus
rhythm-control in persistent atrial fibrillation: The Strategies of Treatment
of Atrial Fibrillation (STAF) study. J Am Coll Cardiol 41: 1690-1696.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Cell Signaling & Damage-Associated Molecular Patterns
- International Journal of Surgery and Invasive Procedures (ISSN:2640-0820)
- Journal of Spine Diseases
- Dermatology Clinics and Research (ISSN:2380-5609)
- Journal of Alcoholism Clinical Research
- International Journal of Clinical Case Studies and Reports (ISSN:2641-5771)
- Journal of Clinical Trials and Research (ISSN:2637-7373)